lb glucose autoclave|autoclaving glucose solution reviews : discounter Autoclave the mixture. 3. Prepare as appropriate: For liquid media. i. Add 50 mL of sterile 40% (w/v) glucose. Mix. ii. Allow to cool before use. For agar plates. iii. While stirring the autoclaved mixture on a magnetic stir plate, add 50 mL of sterile 40% glucose per liter of media. iv.
Excel-healthcare_autoclaves_enigma-17LB-22LB_manual - Free download as PDF File (.pdf) or view presentation slides online.
{plog:ftitle_list}
Afin d’éviter que votre autoclave bois ne vieillisse trop vite avec le temps, nous vous conseillons de le protéger non pas avec une lasure mais un Saturateur Bois Autoclave. Ce produit imprègne votre bois en profondeur ce .
Smaller scale: To achieve a 20 mM concentration of glucose for a known final volume n of SOC, you can calculate the amount of 10% w/v glucose to be added as 0.036n. The . See moreHeart Infusion Agar supplemented with sterile Sheep's Blood. Used for the cultivation of fastidious organisms (Bee gut microbiome species . See moreMaking agar stabs for storage and transport of bacterial strains. Autoclave. Transfer to cryovials before media cools. Can store the vials at . See moreGlucose is a reducing sugar and can react with amino acids in high temperatures (Maillard reaction). The best way of doing it is to filter sterilize and add in the buffer/medium after.
LB media: Lysogeny Broth or LB is the most popular and commonly used medium to culture E. coli. It was first created by Bertani G to culture Shigella indicator strain. LB is a rich medium containing peptone, yeast extract, NaCl and agar (for solid medium).
Autoclave the mixture. 3. Prepare as appropriate: For liquid media. i. Add 50 mL of sterile 40% (w/v) glucose. Mix. ii. Allow to cool before use. For agar plates. iii. While stirring the autoclaved mixture on a magnetic stir plate, add 50 mL of sterile 40% glucose per liter of media. iv.
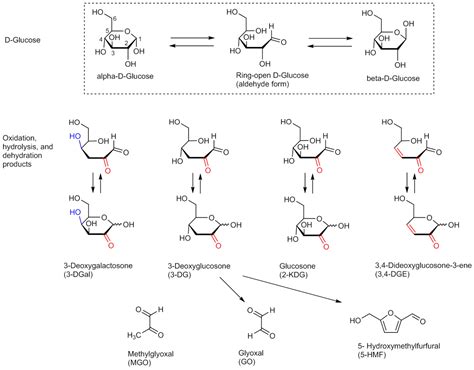
Glucose, used as model sugar in this investigation, was autoclaved for 20,32 or 44 min in volumes of 0.25. 1.2 and 5 1. The rise in temperature of the solutions was monitored by thermocouples. Glucose degradation was estimated by the rate of cyanide-initiated oxygen consumption.
Growth response to d-glucose in exhausted Luria-Bertani medium. Filter-sterilized LB* medium was supplemented with various concentrations of d-glucose, inoculated with MG1655, and incubated for 24 h at 37°C with vigorous aeration. The OD 600 of each culture was measured. The pH of LB* is routinely near 9.Dispense into appropriate containers and sterilize in autoclave at 121°C for 15 minutes. . clear amber, slightly opalescent. Technical information These LB Broths are based on LB Medium described by Miller. LB stands for Lysogeny Broth – and, inexactly, for . the medium can be supplemented with 0.1% glucose or 0.4% glycerol. FT-N1398A .
Pick a new colony, grow overnight in LB. . Cap loosely, wrap with foil, and autoclave. (If you make more than one bottle, the extras go in the water bath till you are ready to pour) . Commonly added to YNB, nitrogen and glucose, at suggested g/L, for a complete yeast media. Mix with stirring for 10-15 minutes, and filter sterilize or .It is always good to autoclave once at 15lbs pressure at 121 0 C for 15-20 min,as it gives the best result. With my personal experience autoclaving PDA, twice has resulted either in no growth of .
LB + Mg + Glucose for better Gram-N growth (agar + antibiotics possible) 1L v1. June 2023; . - If adjusting recipe to 500mL or below 30min autoclave is enough. 3 Let cool .
Glucose (Dextrose) 1 g: Yeast extract: to 1000 ml total: with dI H 2 O: . DO NOT AUTOCLAVE to sterilize, this will change the pH and precipitate out some of the chemicals in the solution. This is the standard 2x concentration minimal media recipe without the addition of Remi’s added trace elements solution. . The salts in LB make it . Autoclaves are generally expensive, energy-hungry beasts that (in my experience) break down a lot so I would be very happy to use them less if I could. Decontamination using microwaves. . Sterilisation using microwaves A 2001 Biotechniques paper by Weiss and Galande showed that LB plates made from microwave-sterilised LB-agar were apparently .40g Glucose. 10mL of 0.2% Adenine. Then: Stir using hot plate w/ stir bar for ~30min. Next, add MQ water up to 2000mL. Pour into 1L glass bottles. Each container should have ~650mL media. Autoclave: ~40min. . Autoclave: ~40min . How to make LB Liquid: For 2L solution: Add:
Use autoclave tape to tape on caps, also put a small bit of autoclave tape on any other items you autoclave. The tape will change color during autoclaving, thus signaling to any future user that the item has really been sterilized. Note: Autoclave tape only .Alternatively, 5g/L NaCl can be used (Lennox formulation) or no salt (-NaCl). The -NaCl (no salt) version of LB should be used when using salt sensitive antibiotics like hygromycin: for example, when cloning into pSS170. (Alternatively, DNA or DNB can be used for hygromycin selection.) To make liquid LB medium, omit the agar and autoclave as above.ATCC medium: 2207 LB medium with 2.0% glucose Tryptone...10.0 g Yeast extract...5.0 g NaCl...10.0 g
#01 LB-Medium Materials: - 10 g/L Tryptone - 5 g/L yeast extract - 10 g/L NaCl - MQ H 2 O Procedure: 1. Mix all components together 2. . For 500 ml stock solution add 100 g glucose to 440 mL MQ H 2 O. Autoclave for 15 min at 121°C. 1.3. 1 M MgSO 4 - 1 M MgSO 4 *7H 2 O → 24.65 g/100 mL For 100 ml stock solution dissolve 24.65 g MgSO 4-7H 2During caramelization the glucose and other sugars that may be present in the media slightly darken and the overall appearance of the media will look more of a dark honey or light brown color. Along with the media itself . ampoules were run in the autoclave for 2 hours at a temperature of 132°C. When removed, the ampoules wereAutoclave the bottled SOB. SOC medium is SOB medium with 20 mM glucose. You can do that by adding 20 ml of 20% glucose into 1 liter of SOB. Make sure everything is sterile though. - As an autoclave will be used for sterilization, have autoclave gloves ready. Materials - 1L Graduated Cylinder - 1L Glass Autoclave Bottle, w/ Cap - Autoclave . Pour a thin layer of LB Agar (~15-20mL) into each plate being careful to not lift the cover off excessively (you should be able to just open up enough to pour).
‘Lysogeny’ or Luria broth (LB) . A combination of salt, magnesium, and glucose stabilizes the bacteria and promotes plasmid uptake, thereby increasing transformation efficiency. 2. . Cover the flask with two layers of aluminum foil and top with a piece of autoclave tape. 2.5.Weigh out 7.5g of agar into a 500ml bottle. Add the water. Cap loosely, pre-warm, and autoclave for 20 minutes, liquid cycle. 9. 4% Agar. For yeast Ingredients: DifcoAgar 10g Distilled H 2 O 250ml Directions: Weigh out 10g of agar into a 500ml bottle. Add the water. Cap loosely, pre-warm, and autoclave for 20 minutes, liquid cycle. 10. 20% Glucose Procedure for 1 L TB. Dissolve and autoclave on liquid cycle, 15–20 min, 15 psi: 12 g tryptone; 24 g yeast extract; 4 mL glycerol (or 8 mL 50% V/V glycerol) (or add after autoclaving) (opt.) 15 g agar (sug.) 5 mL 2 M MgCl 2 or MgSO 4; final 900 mL diH 2 O; Prepare or obtain 100 mL 10× TB buffer (0.17 M KH 2 PO 4 0.72 M K 2 HPO 4) Dissolve and autoclave on liquid .Alternatively, 5g/L NaCl can be used (Lennox formulation) or no salt (-NaCl). The -NaCl (no salt) version of LB should be used when using salt sensitive antibiotics like hygromycin: for example, when cloning into pSS170. (Alternatively, DNA or DNB can be used for hygromycin selection.) To make liquid LB medium, omit the agar and autoclave as above.
Preparation of LB liquid medium: In a 1L autoclave bottle (orange cap), add: 25g LB broth powder 1000mL ultrapure water Swirl to mix. Powder will not dissolve completely, that is ok. Replace the cap to the bottle but leave it slightly loose for pressure equalization to occur. Place a fresh piece of autoclave tape on the top.To sterilise, autoclave the solution on a liquid cycle (20 min at 15 psi). Storage of LB media. Store LB media at room temperature (+15 o C – +25 o C) or in the fridge (+4 o C). Safety. LB media is not classified as a hazardous substance. SOURCE Cold Spring Harbor Protocols. TAGS; Cloning;
Apply autoclave tape to the bottle and autoclave on liquids cycle. Read step 8. CRITICAL Liquids cycle. If adding selectable components or preparing solid medium, . While preparing the LB liquid medium from LB agar, I am facing two problem (not at the same time) 1. Either my medium solidifies at room temperature.
Autoclave for 20 min at 15 psi (1.05 kg/cm 2) on liquid cycle. After Autoclave, cool down the medium to room temperature under laminar airflow chamber. Add MgSO 4, MgCl 2 and Glucose stock solutions till the required working concentrations and mix thoroughly before use. Preparation of SOC agar medium. If you plan to prepare solid media, add . Autoclave sterilization is a method of sterilization that uses heat from pressurized steam to kill microorganisms. Autoclave sterilization is often used to sterilize medical and laboratory .
lambris autoclave
$399.00
lb glucose autoclave|autoclaving glucose solution reviews